


Next: Problems
Up: Chapter 04
Previous: Chapter 04
- #03
- A continuous spectrum means radiation (or ``glow'') is
produced at every wavelength. An absorption spectrum is a continuous
spectrum with depressed or absent radiation at particular wavelengths.
- #04
- Gamma rays are high frequency and so large energy photons
of light. Their energy is so large that when they interact with matter,
they can have strong effects (such as ionization or breaking up
molecules).
- #10
- Normally, electrons of an atom are in their ground state
(or lowest level). An excited atom is when an electron is in a
higher state. Orbitals represent these discrete or quanized levels
where an electron is most likely to be found.
- #15
- H
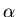 forms when an electron is excited to level 3
in hydrogen and this then falls back down to level 2. The electrons
are generally found in level 1. The energy required of a photon
to move an electron from level 1 to 3 is quite large. The photon
must be of UV light; however, the Sun's photosphere is not hot
enough for UV photons to be plentiful. (The Sun is a yellow star,
not a UV star.) So in spite of all the
hydrogen in the Sun, H
forms when an electron is excited to level 3
in hydrogen and this then falls back down to level 2. The electrons
are generally found in level 1. The energy required of a photon
to move an electron from level 1 to 3 is quite large. The photon
must be of UV light; however, the Sun's photosphere is not hot
enough for UV photons to be plentiful. (The Sun is a yellow star,
not a UV star.) So in spite of all the
hydrogen in the Sun, H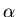 is actually quite weak.
is actually quite weak.
- #19
- The line broadens, because the atoms that create the spectral
line are moving around with the rotation speed of the star. On one side
the gas of the star is coming around from the back and so that part is
moving relatively toward us. The other side moving around to the back and
so that part is moving relatively away from us. Thus the radiation is
Doppler broadened, both in blueshift and redshift. Higher rotation speeds
produce increasingly broader lines.



Next: Problems
Up: Chapter 04
Previous: Chapter 04
Rico Ignace
2004-09-10