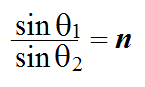Albert Einstein - A Centennial Celebration of His Miraculous Year
The Photoelectric Effect and Light Quanta
Two fundamental properties of optics are the Law of Reflection and the Law of Refraction (also called Snell's Law).
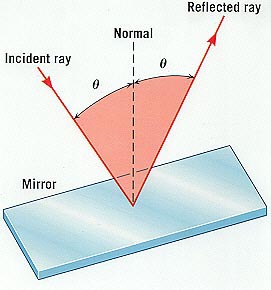
The Law of Reflection, from Joseph Alward's "Properties of Light" webpage.
The Law of Reflection says that if a beam of light falls on a flat reflective surface, then the angle of incidence equals the angle of reflection. This is familiar from our daily experience with mirrors.
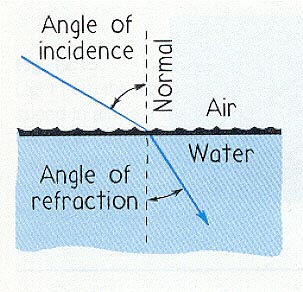
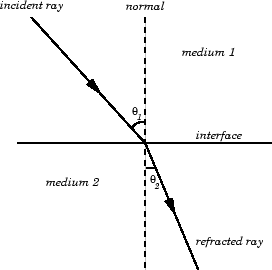
The Law of Refraction, from Joseph Alward's "Properties of Light" webpage.
The Law of Refraction is trickier to state. It describes how a beam of light reacts when it passes from one medium to another (such as from air to water). Quantitatively, it relates the sines of the angle of incidence and the angle of refraction. It states that
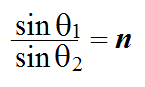 where θ1
is the angle of incidence,
θ2
is the angle of refraction, and n is the index of refraction from one medium to another.
where θ1
is the angle of incidence,
θ2
is the angle of refraction, and n is the index of refraction from one medium to another.
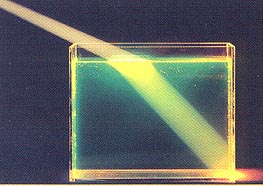
Refracton, from Joseph Alward's "Properties of Light" webpage.
A familiar example of this is the apparent location of a fish underwater when viewed from the bank of a pond.
The question is: "Why does light behave like this?"

Huygens' Principle, named after Christian Huygens (1629-1695), which states that light behaves like a wave. From The MacTutor History of Mathematics archive.
Classically, there are two ideas. One is based on Huygens' Principle, named after Christian Huygens, which states that light behaves like a wave.

James Clerk Maxwell (1831-1879), from The MacTutor History of Mathematics archive.
This idea was developed by James Clerk Maxwell into a mathematically sophisticated theory in the mid 1800's. The refraction is explained by a change in speed of the wave from one medium to another.
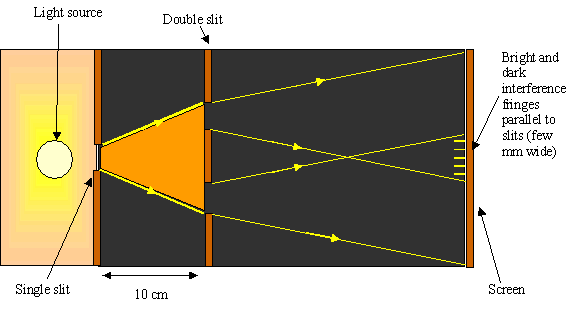
Two slit experiment.
Further evidence for the wave theory is given by the two-slit experiment which shows that light passing through two openings produces two new wavefronts and the waves can interfere with each other.
The other idea is that light comes in little discrete packages, like a barrage of bullets from a machine gun, that comes so fast that it appears to be continuous.

Max Planck(1858-1947), from
Pictures of Famous Physicists.
The first person to take a step in the development of this idea was the German physicist Max Planck in 1900. It was known at the time that if an object with certain ideal properties (called a "black body") is heated to a high temperature, then it emits radiation (in the form of heat and light) in a certain way.
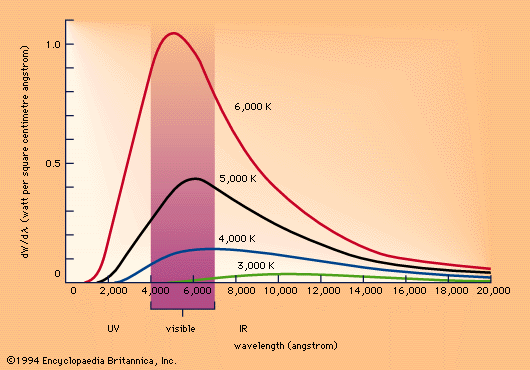
The Planck curve, from Encyclopedia Britannica.
Planck was the first to give a theoretical explanation of this curve. He assumed that the atoms of the black body were tiny electromagnetic oscillators. These oscillators, Planck assumed, cannot take on just any value but can only take on certain discrete values
of the form
E=nhν,
where n is a whole number (called a quantum number,
ν
(nu) is the oscillator frequency, and h us a constant now called Planck's constant. In addition, he assumed that the oscillators do not emit energy continuously, but only in "jumps" or quanta. Based on these two assumptions, Planck was able to theoretically derive the equation for the radiation on the basis of the wave theory.

Einsein at age 21, from Tom Michalik's "Einstein" webpage.
In Einstein's paper on the light quanta, he totally abandoned the wave theory. Instead he assumed the quanta of light were like little particles called "photons." This assumption allowed Einstein to derive Planck's radiation law. his method was both clear and simple and avoided many of the special assumptions that Planck had found it necessary to make in his work.
Einstein's photon idea can also be used to explain the photoelectric effect.
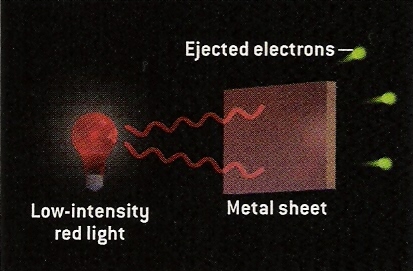
From Scientific American.
If a light shines on a sheet of metal, it will dislodge electrons from the metal. If the light is of low intensity (that is, low brightness) and low energy (say red light), then a few electrons of low energy will be ejected from the metal.
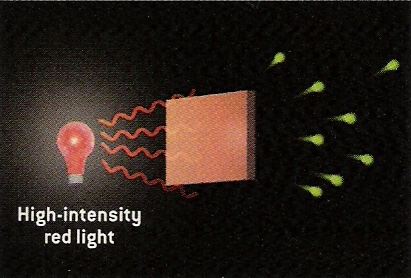
From Scientific American.
By increasing the brightness of the light, we would classically expect the electrons ejected to more energetic. However, this does not occur. Instead, what happens is that some electrons are ejected but they are still of low energy.
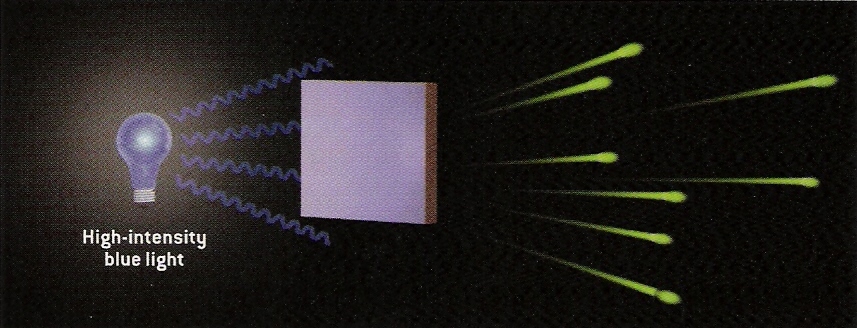
From Scientific American.
Now if we increase the energy of the light, say to blue light, then we find that the ejected electrons are now high in energy. In turn, low intensity blue light will eject a few high energy electrons and high intensity blue light will eject many high energy electrons. The relevant observation is that the energy (or color) of the light determines the energy of the ejected electrons. The intensity of the light does not effect the energy of the electrons, but the number of ejected electrons.
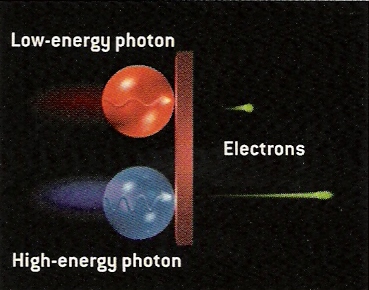
From Scientific American.
All these experimental observations are explained by Einstein's photon idea. The low energy red light hits the metal as low energy photons. These photons "bump" low energy electrons off of the metal. If the intensity of the light increases, then more low energy photons hit the plate and more low energy photons are ejected. By shining the high energy blue light on the plate, high energy photons hit the plate and energetic electrons are knocked off.
Einstein himself considered his paper on these ideas his only revolutionary paper of 1905. The physics community seemed to agree. Einstein was awarded the 1921 Nobel Prize in physics (which he didn't actually receive until November 1922) for his work on the light quanta and the photoelectric effect.
Go to Brownian Motion section.