Research in the McCusker group is focused on two main projects.
Chromium(III) Sensitizers for Oxidative Photochemistry
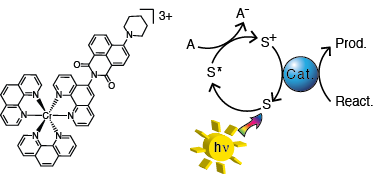
We are developing earth-abundant chromophores for use in oxidative solar fuels photochemistry using Cr(III) polypyridyl complexes. The 2E excited state of Cr(III) is a powerful excited state oxidant, capable of oxidizing known water oxidation catalysts. This family of complexes also have long excited state lifetimes (~100 μs), allowing for bimolecular electron transfer chemistry. Cr(III) polypyridyl complexes primarily absorb in the UV region of the spectrum rather than the visible, so organic light harvesting chromophores are being used to increase the visible light absorption cross-section of the Cr(III) complexes while maintaining the favorable lifetime and excited state reduction potential of the 2E excited state. First, the photophysical and electrochemical properties of these novel Cr(III) complexes will be investigated, and their photosensitizing ability will be tested with known homogeneous water oxidation catalysts.
Metal Dipyrrin Sensitizers for Reductive Solar Fuels Photochemistry
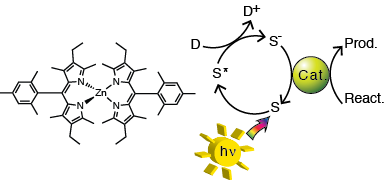
Complexes of the dipyrrin ligand have potential as sensitizers for reductive photochemistry. Dipyrrin ligands have intense visible absorption properties similar to that of the related porphyrins, while being more synthetically accessible. Boron dipyrrin complexes are capable of intramolecular reduction of a cobalt water reduction catalyst, but short excited state lifetimes (ps-ns) make bimolecular electron transfer unlikely. In cases where the dipyrrin ligand is coordinated to a third-row transition metal, dipyrrin based phosphorescence is observed, indicating the long-lived dipyrrin triplet state is formed. First row transition metal complexes of dipyrrin ligands are also known, but the photophysical properties of these complexes have not been widely explored. Dipyrrin complexes with first row metals such as Zn(II), Cu(II), Co(III), or Fe(III) will be synthesized and their triplet state formation will be investigated. Suitable complexes, which form long-lived triplet state in high yields, will be tested with known carbon dioxide or water reduction catalysts.